Research
Click on each research topic to learn more:
Regulation of Angiogenesis by Tumor Suppressor Pathways
Angiogenesis is the physiological process by which new blood capillaries are formed and is an absolutely necessary step in tumour growth in order to allow delivery of oxygen and nutrients. Inhibiting tumour angiogenesis is a promising intervention point for new cancer therapies and identifying novel mechanisms that inhibit angiogenesis is crucial to this endeavor. Angiogenesis is controlled by a balance of pro- and antiangiogenic factors that regulate the growth of endothelial cells, the cellular precursors of the vasculature. As shown on the the home page, endothelial cells are able to form into vessels when placed in the proper context. There is significant evidence that cellular tumour suppressor pathways function in part by limiting angiogenesis using mechanisms that both inhibit the production of proangiogenic factors and actively increase levels of antiangiogenic factors. Our previous studies have demonstrated that tumour cells expressing the p53 tumour suppressor secrete a potent cocktail of collagen-derived angiogenesis inhibitors (see figure below). The production of endogenous angiogenesis inhibitors can have dramatic effects on the growth rates of cancers but the molecular mechanisms that control the biosynthesis of these peptides is poorly understood.
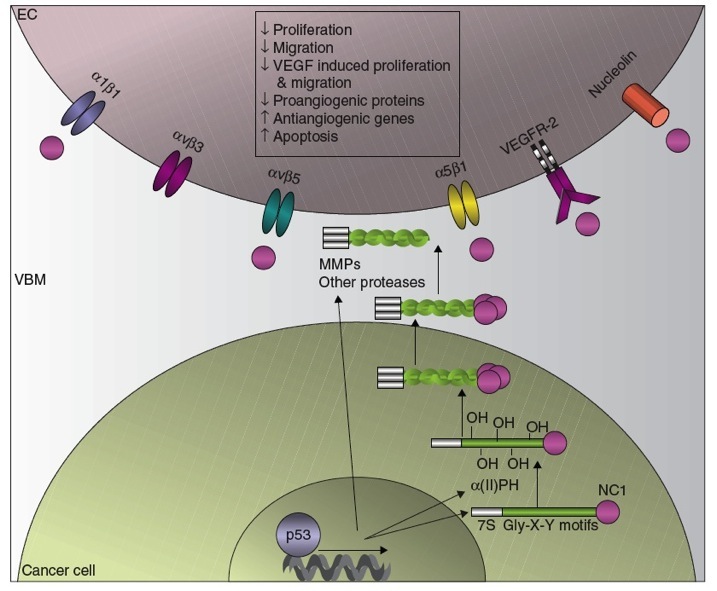
The p53 Tumour Suppressor and Angiogenesis Inhibition
The p53 tumor suppressor protein increases the production of collagen-derived antiangiogenic factors (CDAFs). This occurs by the transcriptional upregulation of (1) the antiangiogenic collagen genes (2) the rate limiting enzyme involved in the synthesis and assembly of collagen, α (II) prolyl-4 hydroxylase ( α (II)PH) and (3) the extracellular proteases necessary for the cleavage of the non-collagenous 1 domains (NC1) such as the matrix metalloprotease (MMPs) and activation of the CDAFs. Once released from their parent collagens, the CDAFs derived from the vascular basement membrane (VBM) bind to their respective receptors on the endothelial cells (EC) and elicit antiangiogenic effects.
Research Topics Under Investigation:
- Which anti-angiogenic factors does p53 modulate in order to inhibit tumour angiogenesis and can such factors be used therapeutically?
- Do other major tumour suppressor pathways such as PTEN also influence angiogenesis and what is/are the mechanism(s)?
- What are the pathways that release endogenous antiangiogenic factors from the extracellular matrix?
The Anaphase Promoting Complex in Cancer and Viral Infection
In order for cell division to occur successfully, a series of events must be perfectly coordinated including DNA synthesis, spindle formation, and nuclear division. Much of the events of cell division are controlled by oscillations of a variety of cyclins that control each step of the cell cycle. Cyclins associate and activate cyclin-dependent kinases (CDKs) and these different oscillating kinase activities are the engines that drive the cell cycle. Cyclin expression undergoes waves of synthesis and destruction resulting in peaks of CDK activity. In order for cells to exit from mitosis, for example, mitotic cyclins must be destroyed and this activity is mediated by the APC/C. The APC/C is a large 1.5 MDa complex composed at least twelve subunits that is required for cell division in all eukaryotes. The function of the APC/C is as an E3 (ubiquitin ligase) that coordinates the degradation of specific substrates via the 26S proteosome at specific points in the cell cycle. The first characterized substrates of the APC/C were the mitotic A- and B- type cyclins.
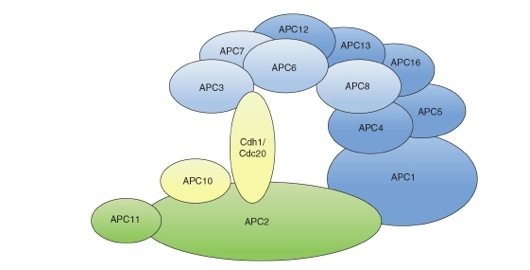
Structural model of the mammalian APC/C. The APC/C consists of a catalytic subcomplex (depicted in green) that can execute substrate ubiquitination and a regulatory subcomplex (depicted in blue) that confers specificity to the ubiquitination process. The two subcomplexes are linked together by the large subunit APC1. APC11 binds directly to the E2 conjugating enzyme and is the subunit that has ubiquitin ligase activity. The subunits APC2 and APC3 are implicated in the binding of the co-activators Cdc20 and Cdh1. Factors involved in substrate recognition are depicted in yellow.
Research Topics Under Investigation:
- Six different classes of viruses interact with the APC/C interfering with normal cell cycle progression and in some cases inducing tumour-selective apoptosis.
- Why do such a wide range of viruses target the APC/C and what role does this play in viral replication?
- Factors targeted by viral proteins are often central nodes in the cellular machinery providing clues for efficient drug targeting (e.g., retinoblastoma (Rb), p53). Tumour cells have been shown to have a higher dependency on APC/C function and experience higher levels of mitotic stress as a result of oncogenic transformation.
- Are tumour cells more susceptible than normal cells to APC/C inhibition and can this be exploited therapeutically?
Biology of Circoviruses
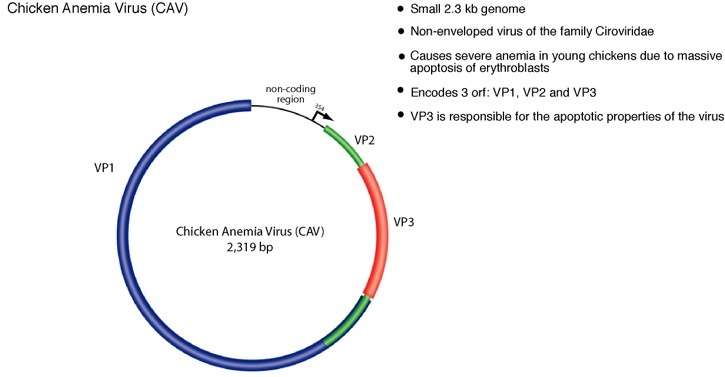
Circoviruses are an unusual and ubiquitous class of animal viruses characterized by circular single-stranded DNA genomes. The circoviruses are non-enveloped, icosahedral viruses and have the smallest genomes of all known animal viruses ranging between 1.7-2.3 kilobases. Circovirus are present in many animals including avian, porcine, bovine, murine, and human hosts2. Although there are no known human pathologies associated with circovirus, outbreaks of avian and porcine circovirus impart recurring economic loss to livestock industries and continue to be a recurring problem worldwide. The avian circovirus, chicken anemia virus (CAV), infects young chickens and induces massive apoptosis in erythroblasts resulting a characteristic anemia and immunosupression. Disease causing circovirus infections are widespread among avian hosts and have been documented in many species including geese, pigeon, gulls, finches and ostrich3. Circoviruses are commonly cause pathologies in wild birds as well and are regarded as an important emerging pathogen with potentially serious effects for domesticated and natural avian fauna.
Recent progress in research activities.
We have been studying the ORF3 protein from CAV as a model for circovirus-induced cytotoxicity. The CAV-ORF3 protein has highly interesting properties that make the protein of interest from a cell biology and virology perspective. In an effort to understand how CAV-ORF3 induces cell death, our group purified the cellular complex associated with the protein using biochemical methods. Using this approach we found that CAV-ORF3 bound to the anaphase promoting complex/cyclosome (APC/C). The APC/C plays a central role in the regulation of cell division and a diverse range of viruses have been shown to bind and deregulate the APC/C, but very little is known about why viruses target this complex. We have demonstrated that CAV-ORF3 induces a potent cell cycle arrest at the G2/M phase of the cell cycle due to the inability of the cells to complete mitosis. Our work suggests that the pathology of the CAV-ORF3 protein in chicken cells may be due to induction of cell cycle arrest of the target cells of the virus
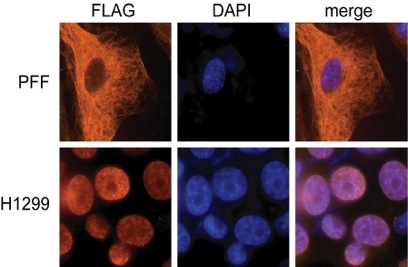
Of particular interest has been the finding that CAV-ORF3 is able to selectively kill cancer cells by a mechanism that is independent of p53. Many types of cancer chemotherapies that function as DNA damaging agents depend on p53 for efficacy. Since p53 is mutated in more than half of all human cancers, these tumors are more resistant to many types of therapy. The study of CAV-ORF3 is therefore a unique system for identifying pathways of apoptosis that are able to kill cancer cells independent of p53. The transformed cell specific killing of CAV-ORF3 is regulated at least in part at the level of subcellular localization (see immunofluorescence experiment on left). When expressed in cancer cells (H1299), CAV-ORF3 localizes to the nucleus, whereas in normal (PFF) cells it is cytoplasmic.
Research Topics Under Investigation:
- What aspect(s) of cancerous cells does CAV-ORF3 sense to cause differential localization and what role does this play in the replication of the virus?
- What triggers the replication of circoviruses in their natural setting?
- Do circoviruses cause disease in humans or modify the progression of other diseases such as cancer?
Role of the G0 → S switch gene (G0S2) in Colorectal Cancer
In Canada, Colorectal cancer (CRC) is the second leading cause of cancer related death. In 2010 an estimated 22,500 Canadians were diagnosed with the disease and 9,100 will die from it. CRC arises from pre-existing benign neoplasms (adenomatous polyps) that can evolve over time into malignant colorectal carcinomas through a stepwise accumulation of mutations. In comparison to other cancer types, the genetic alterations that underlie the progression of CRC is perhaps one the better understood. The best-defined genetic alterations in CRC include activating mutations in the KRAS oncogene and inactivation of the TP53 and APC tumour suppressor genes. Despite the importance of these genetic changes in CRC, only about 10% of all colon cancers are thought to have mutations in these three genes, suggesting that other mechanisms can drive the progression of CRC. In particular, CRC has been associated with the epigenetic effect of CpG island (CGI) methylation. CGIs are short sequences located in or near promoter elements of genes that are rich in the CpG dinucleotide sequence. Methylation on the cytosines of CGIs are correlated with the loss of expression the gene and is thought to contribute to the phenotype of many types of human cancer including CRC. Genes that become methylated and silenced in CRC often encode proteins that mediate critical processes in cancer progression such as cell cycle arrest or apoptosis. The identification and characterization of genes that are silenced in CRC through epigenetic effects is therefore of utmost importance to better understand the mechanism of this cancer. The current proposal aims to characterize a novel tumour suppressor gene, G0S2, which we believe is methylated and silenced in a wide range of human cancers including CRC.
The G0S2 gene encodes a small 12kDa protein localized in the mitochondria and endoplasmic reticulum. More recently, our own research has found that G0S2 can bind and antagonize the activity of the anti-apoptotic protein Bcl-2, which then sensitizes cells to apoptotic stimuli (Welch et al., 2009). Unlike other Bcl-2 inhibiting proteins, G0S2 does not appear to have the hallmark Bcl-2 homology (BH2) domains that leave it in a separate family. We have demonstrated that ectopic expression of G0S2 in a variety of human tumor cells, including colon carcinoma cell line HCT116, results in apoptosis (Welch et al., 2009). Intriguingly, the G0S2 gene has a potent CGI in the promoter region. Work from our lab and others have suggested that the gene is silenced in many types of human cancer including head, neck cancer, squamous lung cancer and glioma. We therefore hypothesize that loss of G0S2 expression may contribute to the aggressive phenotype of metastatic CRC by rendering the cells more resistant to apoptosis. The expression and genomic methylation status of the G0S2 gene have never been studied in CRC. In addition, although the G0S2 gene has several intriguing properties of a tumour suppressor, a direct role for this gene in tumour progression has never been tested using an animal model of cancer.
Research Topics Under Investigation:
- Is the CGI of the G0S2 gene methylated in human CRC and does it correlate with stage of disease?
- What are the effects of G0S2 expression on CRC cell growth in vitro and in vivo?
- Does the G0S2 gene play a role in the progression in mouse models of intestinal cancer?
Research in our lab would not be possible without funding from our sponsors.





